All published articles of this journal are available on ScienceDirect.
Deep Learning Model to Predict In-hospital Mortality of Newborns during Congenital Heart Disease Surgery
Abstract
Purpose:
Many parents are concerned about the cost of saving their child's life. The operation's cost depends on the pathology's nature and the chosen clinic's class. The human body functions as a single system where each organ performs its function. The heart is the main organ of the circulatory system and is responsible for filling all the blood vessels in the body. Surgery in 72% of diseases gives a chance for a complete recovery of the child. Its success depends on timing.
Methods:
In this paper, an AI-induced deep learning model has been proposed to predict in-hospital mortality of newborns in congenital heart disease surgery. If the structure of the heart chambers or large vessels is different from normal, this indicates a defect. Heart disease is a disease caused by changes in the structure of valves, septa or blood vessels. These defects can lead to poor blood circulation in the body and depending on the affected area. Almost all heart defects are curable, often with surgery. Modern medicine has many successful cases of surgical treatment of heart defects in adults and children.
Results:
The proposed model reached 68.41% of training accuracy and 84.83% of testing accuracy, 83.44% training false discovery rate and 85.18% testing false discovery rate, 78.48% training false omission rate and 84.72% testing false omission rate, 70.26% training Positive likelihood ratio and 82.40% of testing positive likelihood ratio and 80.15% of training negative likelihood ratio and 82.97% of testing negative likelihood ratio.
Conclusion:
With the development of modern surgery, early correction of CHD is possible even in low birth weight and premature babies. During surgery, the heart and lungs are cut off from the bloodstream, during which it is enriched with oxygen, which is distributed throughout the body. If the case is complicated, additional surgery may be required over a period of several months to 1 year from the previous surgery.
1. INTRODUCTION
The mortality rate of newborns undergoing surgery for congenital heart disease is an important indicator of the quality of care they receive [1]. In-hospital mortality of newborns during congenital heart disease surgery can provide valuable insight into the efficacy of the medical team, the availability of resources and the overall quality of care [2]. High mortality rates in newborns undergoing congenital heart disease surgery are mainly due to the complexity of the disease and the difficulty in detecting and treating defects in the early stages of life [3]. In-hospital mortality rates of newborns in congenital heart disease surgery have been found to be higher than in other pediatric surgical procedures [4]. This is mainly because of the urgency of the surgery, the complexity of the procedure, and the increased risks associated with surgery on a newborn [5]. In-hospital mortality of newborns in congenital heart disease surgery can be reduced by providing a multidisciplinary approach to care, involving the use of experienced pediatric cardiac surgeons, anesthesiologists and other specialists [6, 7]. The availability of necessary resources to support the surgery is also important. These resources include adequate staffing, specialized training and equipment, and access to an appropriate environment to perform the surgery [8]. In-hospital mortality rates of newborns in congenital heart disease surgery can also be reduced by ensuring that the patient and family are adequately informed and educated about the risks and benefits of the surgery [9, 10]. This is especially important for parents of newborns, as they are likely to be more concerned about the potential outcomes of the procedure [11].
The survival percentage of congenital heart surgery for newborns depends on several factors, including the type of surgery, the severity of the condition, and the experience of the surgeon. Generally speaking, the survival rate for newborns undergoing congenital heart surgery is between 70 and 90 percent [12]. This is largely due to the fact that the condition is frequently diagnosed before birth and the surgery is performed shortly after the baby is born. Additionally, the advancements in medical technology and the experience of the surgical team can also contribute to improved outcomes [13]. When the surgery is performed on a newborn, the survival rate is typically higher if the surgery is performed on an older child or an adult. This is because newborns tend to have smaller hearts, which makes the surgery less complex, and the chances of post-operative complications are lower [14]. Additionally, newborns also have a stronger immune system and greater ability to recover from the surgery. Despite the relatively high survival rate, there are still risks associated with congenital heart surgery for newborns. Some of the potential complications include bleeding, infection, arrhythmias, and stroke [15]. It is important to discuss these risks with the doctor before the surgery is performed. The survival rate of congenital heart surgery for newborns is generally good. However, due to the associated risks, it is important to discuss the potential complications with a doctor before the surgery [16].
Congenital heart disease (CHD) is a condition in which the structure of the heart is abnormal. This can include malformations of the heart valves, walls, or blood vessels. It is a common birth defect, affecting around one in every hundred babies [17]. Surgery is often necessary to correct the defect, as it can cause heart failure and other serious complications if left untreated. The severity of the surgery varies with each individual case. Depending on the type and complexity of the defect, the procedure may involve several steps [18]. These can include closing holes in the heart, repairing or replacing valves, or reconstructing the heart’s walls or vessels. The surgery often requires the use of cardiopulmonary bypass (CPB) to support the heart during the operation and can involve a lengthy recovery period. While some cases are minor and can be managed with medication alone, others require surgery to correct the defect [19]. While CHD surgery is a relatively safe and routine procedure, it is still a major surgery and carries some risks. The best way to predict the risk of CHD surgery for a newborn is to consult a pediatric cardiologist. This specialist can evaluate the patient’s medical history and perform tests to determine the severity of the defect and the patient’s health [20, 21]. They can then recommend the best course of action for the patient, including whether surgery is necessary and, if so, the risks associated with the procedure. In addition to consulting with a pediatric cardiologist, parents can also look into the experience and success rates of the surgeon and hospital they are considering. It is important to make sure that the surgeon performing the operation has extensive experience in CHD surgery and a good track record of successful operations [22]. Parents should also inquire about the hospital’s infection control protocols and ask how the hospital is handling the COVID-19 pandemic [23]. The main contribution of this research paper has provided the following:
- It is important for parents to discuss the risks of CHD surgery with their doctor and to ask any questions they may have. The deep learning models can provide parents with the necessary information to make an informed decision about the best course of action for their newborn.
- The risk of CHD surgery for newborns depends on a variety of factors, including the type of defect, the size of the defect, and patient’s health. The most common risks associated with CHD surgery are infection, bleeding, and heart failure. Other risks include stroke, arrhythmia, and pericardial effusion.
- The severity of congenital heart disease surgery for newborns is often hard to comprehend. The complexity of the surgery and the associated risks are difficult for many to grasp. It is important to understand the severity of the condition, the risk of surgery, and the potential long-term impacts of the procedure.
The remaining part of the paper has been organized as follows. Section 2 provides a detailed literature analysis of existing works. Section 3 provides a detailed explanation of the proposed methodology for the proposed AI induced deep learning model. Section 4 provides detailed information about the proposed algorithm and flowchart and Section 5 gives a comparative analysis of the proposed model. Section 6 illustrates the comparative analysis between the proposed and existing models. Finally, section 7 provides the conclusion and future scope of the proposed deep learning model.
2. LITERATURE ANALYSIS
Cardiac rehabilitation is divided into several phases. The first lasts three to six months. During this period, a person is taught special rehabilitation exercises. A nutritionist explains new principles of nutrition, and a cardiologist observes positive changes in the functioning of an organ, a psychologist helps to adapt to new living conditions.
Van den Eynde et al. [20] discussed a central place in the program given to correcting physical activity because it is necessary to be in good condition not only the heart muscle but also the heart vessels. Physical activity helps control blood cholesterol levels, blood pressure and weight loss. Lying down all the time after surgery can be harmful. Zeng et al. [21].
discussed the heart's need to get used to the regular rhythm of life, and to do this, it is precisely measured physical activity like running, exercise bikes, swimming, and walking. Basketball, volleyball and strength training equipment are contraindicated. Jiwani et al. [22] explained that heart disease in children is a pathology in which the damaged valves, septa, and openings between the heart chambers and blood vessels provoke a violation of blood flow through the inner heart vessels. It is no secret that not everyone enjoys such heart surgeries due to medical conditions. This fact has frustrated medical scientists, so for years they have been looking for ways to increase patient survival. In the end, there was such a technique of surgical intervention as “bloodless surgery”. Lin et al. [23] discussed an aortic valve stenosis was diagnosed and the patient was considered hopelessly ill. This valve should have been replaced, but for various reasons, the probability of the patient's survival was not very high. Cardiac surgery without the use of a heart-lung bypass machine, or “off-pump” surgery, is possible for newborns and is becoming increasingly common.
Park et al. [24] explained that the type of surgery is less invasive and eliminates the risks associated with using a bypass machine, such as bleeding, infection and organ damage. Off-pump surgery is also much less expensive than traditional on-pump surgery. Hu et al. [25] discussed that the surgeon performs off-pump surgery by placing sutures or patches directly on the heart tissue, allowing the heart to remain beating during the procedure. This is done with the help of an endoscope or a tiny camera, which is inserted through a small incision in the chest. Qi et al. [26] discussed that in certain cases, off-pump surgery is not feasible, and a heart-lung bypass machine may be required. However, this type of surgery is usually used only when the defect is too complex to repair without the help of the bypass machine. Bertsimas et al. [27] discussed that the prosthesis was inserted into the patient's aorta without breast incisions (through a puncture in the thigh). Then, using a catheter, the valve was directed in the right direction - to the heart. The special manufacturing technology of the prosthesis allowed it to roll into a tube when inserted, but once it entered the aorta, it opened to its normal size.
Lei et al. [28] expressed that these surgeries are for the elderly and some children who cannot undergo full-scale surgical intervention. When heart failure is found in children, surgical treatments are mandatory. If we talk about the last stages of the disease, drugs and diet cannot handle it. Comoretto et al. [29] discussed that with the development of new technologies, surgical treatment is available not only for children from one year of age, but also for older children. When an acquired heart defect is diagnosed, the main goal is surgical intervention - to preserve the effectiveness of a person's heart valves. In cases of birth defects or irreparable disorders, valve replacement is required. Peterson et al. [30] discussed that prosthetics can be made from mechanical or biological materials. In fact, the cost of operation depends on this. Open heart surgery is performed under artificial circulation. Rehabilitation after such surgical intervention is long and requires patience and, most importantly, attention to the little patient. Bobillo-Perez et al. [31] confirmed that symptoms of heart disease in children are a reason to start immediate treatment to prevent irreversible changes in the organ. Doctors do not always turn to surgical procedures - some patients don't need surgery, at least for a while. What is really needed is to prevent the disease that provoked the disease we are considering. Garcia-Canadilla et al. [32] discussed that if heart failure is diagnosed in children, treatment assumes an effective daily regimen. Such children should definitely lead an active and mobile lifestyle with moderate physical activity. But too much work - physical or mental - is categorically contraindicated. Ruiz et al. [33] expressed that an aggressive and strenuous sports should be avoided, but jogging, roller skating or cycling and so on can be useful. It is possible that drug therapy will be needed to help eliminate heart failure. Diet plays an important role in treating the disease. Table 1 provides a comprehensive analysis of the existing research works.
| Authors | Research Highlights |
|---|---|
| Van den Eynde et al. [20] | A central place in the program is given to correcting physical activity, because it is necessary to be in good condition not only the heart muscle, but also the heart vessels. Physical activity helps control blood cholesterol levels, blood pressure and weight loss. |
| Zeng et al. [21] | The heart needs to get used to the regular rhythm of life, and to do this it is precisely measured physical activity like running, exercise bikes, swimming, walking. |
| Jiwani et al. [22] | Heart disease in children is a pathology in which the damaged valves, septa, openings between the heart chambers and blood vessels provoke a violation of blood flow through the inner heart vessels. It's no secret that not everyone enjoys such heart surgeries due to medical conditions. |
| Lin et al. [23] | An aortic valve stenosis was diagnosed and the patient was considered hopelessly ill. This valve should have been replaced, but for various reasons, the probability of the patient's survival was not very high. |
| Park et al. [24] | The type of surgery is not only less invasive, but it also eliminates the risks associated with using a bypass machine, such as bleeding, infection and organ damage. |
| Hu et al. [25] | The surgeon performs off-pump surgery by placing sutures or patches directly on the heart tissue, allowing the heart to remain beating during the procedure. This is done with the help of an endoscope or a tiny camera, which is inserted through a small incision in the chest |
| Qi et al. [26] | In certain cases, off-pump surgery is not feasible, and a heart-lung bypass machine may be required. However, this type of surgery is usually used only when the defect is too complex to repair without the help of the bypass machine. |
| Bertsimas et al. [27] | Using a catheter, the valve was directed in the right direction - to the heart. The special manufacturing technology of the prosthesis allows it to roll into a tube when inserted, but once it enters the aorta, it opens to its normal size. |
| Lei et al. [28] | When heart failure is found in children, surgical treatments are mandatory, if we talk about the last stages of the disease, drugs and diet cannot handle it. |
| Comoretto et al. [29] | When an acquired heart defect is diagnosed, the main goal is surgical intervention - to preserve the effectiveness of a person's own heart valves. In cases of birth defects or irreparable disorders, valve replacement is required. |
| Peterson et al. [30] | Open heart surgery is performed under artificial circulation. Rehabilitation after such surgical intervention is long and requires patience and, most importantly, attention to the little patient. |
| Bobillo-Perez et al. [31] | The confirmed symptoms of heart disease in children are a reason to start immediate treatment to prevent irreversible changes in the organ. |
| Garcia-Canadilla et al. [32] | If heart failure is diagnosed in children, treatment assumes an effective daily regimen. Such children should definitely lead an active and mobile lifestyle with moderate physical activity. |
| Ruiz et al. [33] | Aggressive and strenuous sports should be avoided, but jogging, roller skating or cycling and so on can be useful. It is possible that drug therapy will be needed to help eliminate heart failure. |
Diagnosing congenital heart disease in newborns is a process that can be more accurate and precise using deep learning models. A deep learning model can predict in-hospital mortality of newborns undergoing congenital heart disease surgery. Such a model would require a taxonomy of machine learning algorithms, such as convolutional neural networks, recurrent neural networks, and support vector machines.
- A convolutional neural network could be used to process images, which can provide valuable insight into the condition of an infant’s heart, such as the size of the left ventricle or the severity of the condition. Information collected through these images can be used for predictive analytics, such as providing more accurate predictions about an infant’s survival rate under a given condition.
- Recurrent neural networks can then process the mass of data from the images and patient histories to generate more accurate predictions. These networks can consider multiple variables, which may be difficult to spot with the naked eye or have far greater importance than previously assumed.
- The support vector machines can then accurately determine what variables should be used for predictions and which should be omitted. These machines can identify patterns indicating a higher mortality risk in specific procedures, illnesses, or patient conditions.
The taxonomy of the deep learning model for predicting in-hospital mortality in newborns with congenital heart disease would include convolutional neural networks, recurrent neural networks, and support vector machines to correctly identify valuable data, process it, and make accurate predictions. Such models could benefit the healthcare industry by providing accurate predictions and improving healthcare outcomes.
Deep learning models have become increasingly popular in medical research. The main novelty of the proposed deep learning model is to predict in-hospital mortality in newborns undergoing congenital heart disease surgery. Understanding the specific deep learning model architecture employed for predictive mortality is essential. This model type is typically referred to as a neural network, consisting of an input layer, several hidden layers, and an output layer. The input layer comprises a set of features related to the patient and their medical history, such as age, sex, type of congenital heart disease, etc. The hidden layers are a set of nodes connected in a network-like structure that process the input data and function as the “thinking” component of the model. The output layer passes the processed information to an activation function, determining the patient's mortality risk. The most recently developed deep learning model for predicting mortality in newborns with congenital heart disease relies heavily on convolutional neural networks (CNNs). CNNs are ideal for analyzing a large set of data points in order to distinguish patterns between patients. It is done by combining multiple convolutional (filtering) layers and pooling (down sampling) layers to classify patient data accurately. It is advantageous for predicting newborn mortality, as it allows the model to better account for genetic heart disease variability and better discriminate between observations in the data. One approach to improving the accuracy of the deep learning model is to use a more extensive set of diverse training data. This larger dataset should represent a large variety of patient backgrounds and conditions to ensure that the model can more accurately encompass the diverse range of outcomes associated with the disease. The choice of feature inputs used to train the model should be carefully considered. The model can be trained with better accuracy by selecting the most significant and meaningful features. The architecture employed by the CNN should also be optimized for the task at hand. It can be done by tuning the different hyperparameters of the network, such as the number of layers and number of neurons contained within each layer. The regularization techniques, such as dropout and batch normalization, can further reduce the risk of overfitting the model to the training data. The deep learning models have proven to be highly effective for predicting in-hospital mortality in newborns with congenital heart disease. This essay describes the taxonomy of the deep learning model used for this purpose and how the model's accuracy might be improved by using larger data sets, selecting the essential features, and optimizing the model architecture.
3. METHODOLOGY
The aim of this study is to develop a deep learning model based on artificial intelligence (AI) for predicting in-hospital mortality of newborns in congenital heart disease (CHD) surgery. Currently, there is a lack of predictive models that can accurately estimate the risk of in-hospital mortality for newborns undergoing CHD surgery.
3.1. Symptoms Analysis
Aortic stenosis takes a long time without symptoms; the first complaints begin when the valve opening is reduced to more than 2/3 of the average level. These are compressive pains in the chest during physical exertion, fainting, and dizziness. Later, cardiac asthma, dyspnoea at rest, fatigue and weakness may develop. Further growth causes swelling of the legs and pain in the right hypochondrium. The pale or blue skin, swelling of jugular veins. Systolic fibrillation a la, weakening of the first and second tones, systolic murmur, increasing in the upper right side, will pay attention if it holds the breath while breathing. The pulse is rare and weak.
3.2. Preprocessing
Heart defects in children are accompanied by specific symptoms that you should be aware of and sound the alarm if the child has them. During a routine check-up, the pediatrician can ask the sick child. After their diagnosis, the attending physician should prescribe an ultrasound scan. But the diagnosis of “heart disease” may not be confirmed because, in growing children, functional heart murmurs are the norm. In the first months of life, the physical growth of babies is very intensive; every month, they need to gain at least 400 grams of weight. If this does not happen, you should go directly to a cardiologist because the lack of weight gain is one of the main symptoms of heart problems. Lethargy and tiredness in the child is an obvious signal of health problems. If shortness of breath is added to all this, the risk of hearing an unpleasant diagnosis increases.
3.3. Feature Selection
During pregnancy, it is almost impossible to establish the development of the disease in a child. During a transvaginal ultrasound, an experienced specialist may notice some changes in the work of the child's heart, but much pathology has not yet appeared at this time. The categories of women at risk were identified above - such mothers should take the initiative and undergo a transabdominal ultrasound in the 20th week of pregnancy. After children are born, tests for heart diseases are not included in the list of mandatory tests and examinations. And parents themselves do not take the initiative and do additional diagnostic procedures. The very beginning, the symptoms of the disease do not make themselves felt. Although the child feels that something is wrong with him, he cannot explain it. As parents are very busy with daily work, they regularly take their children for some exams.
3.4. Feature Extraction
Newborns usually only have an ECG and a few more tests, which is usually the end of the diagnosis. However, an electrocardiogram at such a young age cannot detect congenital heart disease. If an ultrasound scan is performed, the disease can be determined at an early stage. It depends on the experience of the ultrasound specialist. It is better to repeat this method in several clinics at the same time, especially if heart failure is suspected.
3.5. Classification
If the symptoms of heart disease in children bring you to the doctor's office and the diagnosis is confirmed, this is not a reason for despair. The course of the disease does not always lead to tragic consequences. For example, grade I and II left atrioventricular valve insufficiency allows people to live 20 to 40 years without surgery while maintaining a certain level of function. But the same diagnosis, but already III and IV degrees, accompanied by shortness of breath during physical exertion, edema in the lower legs, and liver problems, requiring immediate treatment and urgent surgical intervention.
3.6. Detection
The most popular and effective diagnostic method is ECHO-cardioscopy with Doppler. It allows not only to detect a defect but also to assess its severity. They conduct an ECG and daily (Holder) ECG - they show heart rhythm and phono- cardiography to determine heart sounds and murmurs. This clarifies the type of defect using radiography. Acquired heart defects in children are usually due to a malfunctioning valve system. This problem is solved by surgery and valve replacement helps to return to the previous active life.
3.7. Treatment Suggestions
By improving the quality of care and reducing the mortality rate of newborns in congenital heart disease surgery, the medical team can ensure that the best possible outcome is achieved for the patient. The treatments of congenital heart surgery for newborns are:
- Open heart surgery - This procedure involves making an incision in the chest and using surgical tools to make repairs to the heart.
- Balloon angioplasty - This procedure involves using a catheter to place a small balloon into a blocked blood vessel and then inflating the balloon to open up the vessel and improve blood flow.
- Catheterization - This procedure involves inserting a small tube into the heart to diagnose or treat certain heart conditions.
- Pacemaker implantation - This procedure involves surgically placing a small device called a pacemaker in the chest to help control the heart rate and rhythm.
- Defibrillator implantation - This procedure involves surgically placing a small device called a defibrillator in the chest to help correct abnormal heart rhythms.
- Arterial switch operation - This procedure involves switching the locations of the aorta and pulmonary artery to correct certain congenital heart defects.
This will ultimately lead to improved long-term outcomes for the patient, their family, and the medical team. Congenital heart disease (CHD) is a common birth defect that occurs in 1 out of every 100 newborns.
4. PROPOSED MODEL
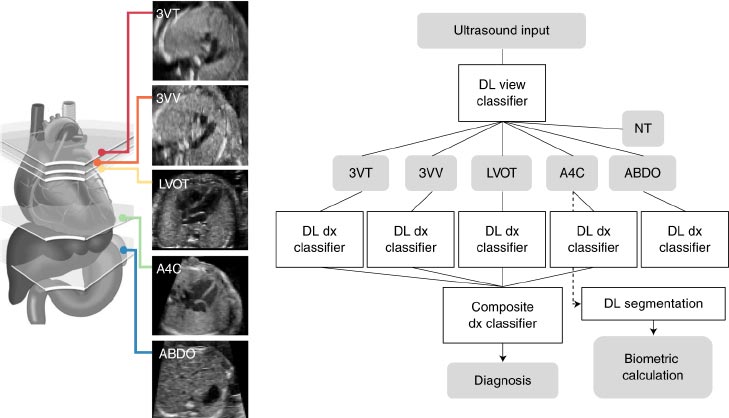
The proposed model will be based on a combination of clinical, laboratory, and imaging information extracted from patients’ medical records. First, we will collect a dataset of newborns with CHD who have undergone surgery. Fig. (1) shows the proposed model diagnosis detection.
This dataset will include information such as the type of CHD, age, gender, preoperative and postoperative laboratory values, preoperative and postoperative imaging findings, and the mortality outcome. We will then develop a deep learning model using the collected dataset. The model will take the collected data as inputs and use AI algorithms to analyze the data and produce a prediction of in-hospital mortality. Algorithm 1 shows the AI induced deep learning algorithm structure.
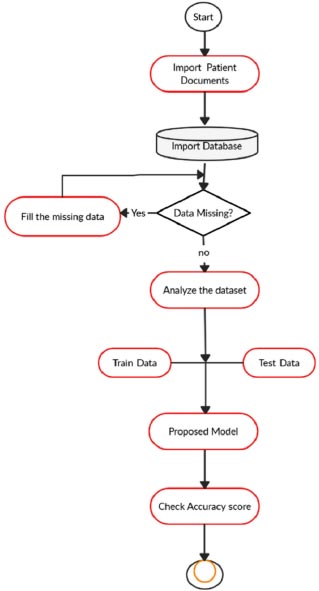
The model will be evaluated by comparing the predicted mortality with actual mortality outcomes in the dataset. We will also use cross-validation to further assess the model’s accuracy and reliability. We will also perform sensitivity and specificity analysis to ensure that the model is not over- or under-predicting mortality. Fig. (2) shows the proposed flow chart.
Finally, we will assess the model’s performance in an external validation set. The proposed deep learning model will provide a valuable tool for clinicians to estimate the risk of in-hospital mortality for newborns with CHD undergoing surgery. We believe that the model can provide a more accurate and reliable prediction of mortality and improve the quality of care for these newborns.
| Algorithm 1: AI induced deep learning algorithm. | |
|---|---|
| 1. | Start |
| 2. | Import the CHD documents; |
| 3. | Create the database as per the documents; |
| 4. | If (data = available) |
| 5. | Then Analyze the data set; |
| 6. | Analyze the training data; |
| 7. | Analyze the testing data; |
| 8. | Compare the training and testing data; |
| 9. | Check the accuracy score; |
| 10. | Else fill the missing data; |
| 11. | Go to step 3; |
| 12. | Stop |
5. RESULTS AND DISCUSSION
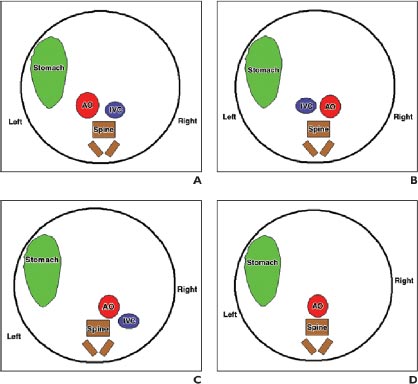
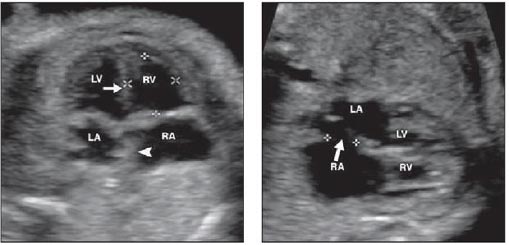
In the compensation phase of mitral insufficiency, people do not feel problems; however, when the condition worsens, shortness of breath may occur (first during physical activity, then at rest), palpitations, dry cough, and chest pains (in the area of the heart). Later, there is swelling of the lower extremities and pain in the right hypochondrium. Doctors on examination reveal cyanosis of the skin and swelling of the veins in the neck. A systolic murmur is observed on auscultation, weakening or absence of eye tone. There were no characteristic changes in pulse rate and blood pressure. Fig. (3) shows the various atrial and situs arrangement.
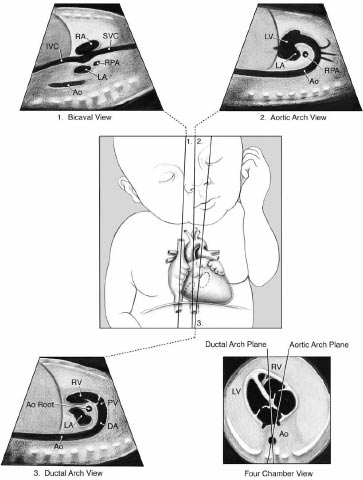
Axial pictures taken at the level of the lower thoracic spine are depicted in the diagrams. The aorta (AO) is situated to the left of the inferior vena cava in that section-A, In situ solitus (IVC). The location of AO is to the right of IVC in section B, In Situ Inverses. The AO and IVC are situated on the same side of the spine in that section C's right isomerism. IVC cannot be seen since it is disrupted in that section-left D's isomerism, where AO is centrally placed. Fig. (4) shows the different views of newborns in congenital heart disease surgery.
The three-dimensional transbicaval view is a novel imaging technique used to assist with the diagnosis and management of congenital heart disease. This view allows for the visualization of the entire cardiac anatomy from the left side of the heart to the right side of the heart. This view is obtained by placing a transbicaval catheter in the superior vena cava and aorta and then taking a two-dimensional (2D) cross-sectional view. The transbicaval view provides detailed anatomical information to the surgeon, including the size and shape of the cardiac chambers, the presence of any obstructive lesions, the location of any shunts, the anatomy of any associated vessels, and the anatomy of the great vessels. This view can help the surgeon to better plan and execute the surgery and can also be used to detect any complications or issues that may arise during the procedure.
Aortic arch view is a type of imaging technique used in congenital heart disease surgery. It is used to visualize the aorta, the aortic arch, and other structures of the heart in order to guide the surgery. The technique is typically used in combination with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI). During the procedure, a special catheter is inserted through a vein in the neck and maneuvered into the aorta. The catheter is then used to inject a contrast material, which allows the surgeon to see the aortic arch and other structures in greater detail. The technique can help surgeons plan and execute complex operations more accurately and safely.
The Sino-tubular ductal arch view is a surgical technique used in the repair of congenital heart disease. It is a three-dimensional visualization of the aortic arch and its branching vessels. The view is created by placing a thoracoscope along the aorta, allowing for direct visualization of the aortic arch and its branches. This technique allows for a more detailed assessment of the anatomy and is often used in the repair of complex congenital heart defects. It can also be used to detect and correct anomalies such as coarctation of the aorta, interrupted aortic arch, and other complex anomalies.
A four chamber view in congenital heart disease surgery is an imaging technique used to observe the four chambers of the heart, which are the left and right atria and left and right ventricles. It is used to diagnose and assess the severity and type of congenital heart disease, as well as to plan and guide surgical procedures. The four-chamber view can be obtained through echocardiography, cardiac MRI, and computed tomography (CT). Fig. (5) shows the ECG diagrams of Various atrial and situs arrangements.
- Preoperative Weight: Low preoperative weight is associated with increased mortality risk in newborns with congenital heart disease undergoing surgical repair.
- Type of Surgery: Certain types of congenital heart disease surgery, such as single-ventricle palliation and complex intracardiac repair, are associated with higher mortality risk.
- Co-morbidities: Co-morbidities such as respiratory and renal disorders are associated with increased mortality risk.
- Duration of Surgery: Longer duration of surgery is associated with increased mortality risk.
- Postoperative Complications: Postoperative compli- cations such as bleeding, infection, and respiratory distress are associated with increased mortality risk.
- Age: Younger infants have a higher mortality risk than older infants.
- Preoperative Severity: Patients with more severe preoperative conditions have a higher mortality risk.
With mitral stenosis, new ones are added to the above complaints. A person standing up suddenly may develop cardiac asthma. The cough is dry, there may be some phlegm, and hemoptysis occurs. There is hoarseness of voice and increased fatigue. Often, against the background of heart pain and tachycardia, arrhythmia begins. BPS is otherwise known as valvular defects: These diseases affect the heart valves. The reasons for their development are infections, inflammation, autoimmune processes, and an overload of heart chambers. Diseases due to development are rheumatism (up to 30-50% of all mitral stenosis - consequences of rheumatism), atherosclerosis, bacterial endocarditic, syphilis (included in the list of syphilitic heart diseases) and other diseases. If the defects are expressed very little, they are not clinically manifested. In the stages of degeneration, hemodynamic disturbances appear, characterized by shortness of breath during physical exertion, blue skin, swelling, tachycardia, cough, and sternum pain. Table 2 shows the symbolic representation table.
| Symbol | Explanation |
|---|---|
| CHD | Congenital Heart Disease |
| PTI | Positive True Identification |
| NTI | Negative True Identification |
| PFI | Positive False Identification |
| NFI | Negative False Identification |
| FDR | False discovery rate |
| FOR | False omission rate |
| PLR | Positive likelihood ratio |
| NLR | Negative likelihood ratio |
6. COMPARATIVE ANALYSIS
The proposed AI induced deep learning model (AIDLM) has been compared with the existing Machine Learning Model for Predicting Risk (MLMPR) and Deep Learning for improved risk prediction (DLIRP).
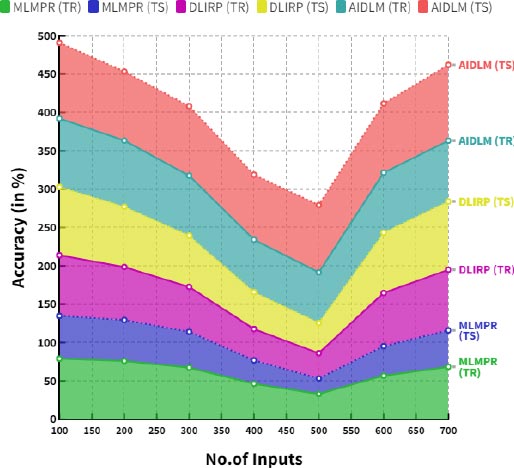
6.1. Computation of Accuracy (A)
The accuracy of newborns in diagnosing congenital heart disease is generally good but can vary depending on the speci- fic condition. Many conditions can be detected by a physical exam, but more complex conditions may require additional testing, such as an echocardiogram or electro- cardiogram. The accuracy of the diagnosis is also affected by the skill and experience of the healthcare provider performing the exam.
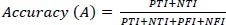 |
(1) |
Table 3 provides the comparison of accuracy (training and testing) between the existing and proposed models.
Fig. (6) demonstrates the comparison of accuracy between the existing and proposed models. In a comparison tip, the proposed AI induced deep learning model (AIDLM) reached 68.41% of training accuracy and 84.83% of testing accuracy. In the same range, the existing Machine Learning Model for Predicting Risk (MLMPR) has reached 46.29% of training accuracy and 30.49% of testing accuracy and Deep learning for improved risk prediction (DLIRP) has obtained 40.88% of training accuracy and 47.99% of testing accuracy.
| No. of Inputs | MLMPR (TR) | MLMPR (TS) | DLIRP (TR) | DLIRP (TS) | AIDLM (TR) | AIDLM (TS) |
|---|---|---|---|---|---|---|
| 100 | 79.00 | 55.81 | 79.00 | 89.03 | 89.20 | 98.77 |
| 200 | 75.96 | 53.45 | 69.14 | 78.42 | 86.33 | 89.99 |
| 300 | 67.35 | 46.79 | 58.42 | 66.87 | 78.24 | 90.44 |
| 400 | 46.29 | 30.49 | 40.88 | 47.99 | 68.41 | 84.83 |
| 500 | 32.96 | 20.17 | 32.98 | 39.48 | 65.87 | 87.80 |
| 600 | 56.74 | 38.58 | 69.31 | 78.59 | 78.25 | 90.14 |
| 700 | 68.37 | 47.59 | 78.99 | 89.02 | 79.20 | 98.76 |
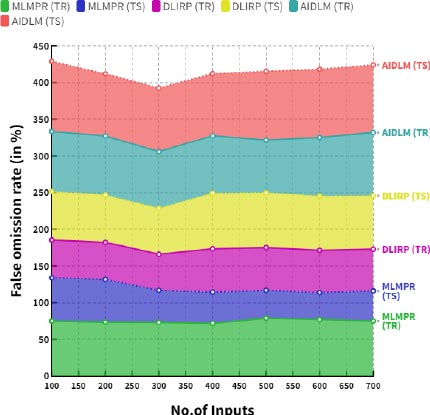
6.2. Computation of False Discovery Rate (FDR)
The false discovery rate for newborns with congenital heart disease is difficult to quantify due to the rarity of the disease and the heterogeneity of diagnoses. However, a recent systematic review concluded that the false positive rate for echocardiography diagnoses of congenital heart disease in newborns is approximately 5%.
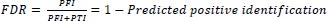 |
(2) |
Table 4 provides the comparison of the false discovery rate (training and testing) between the existing and proposed models.
Fig. (7) demonstrates the comparison of false discovery rates between the existing and proposed models. In a comparison tip, the proposed AI induced deep learning model (AIDLM) reached 83.44% training false discovery rate and 85.18% testing false discovery rate. In the same range, the existing Machine Learning Model for Predicting Risk (MLMPR) has reached 62.68% of training false discovery rate and 52.34% of testing false discovery rate and Deep learning for improved risk prediction (DLIRP) has obtained 54.31% of training false discovery rate and 76.31% of testing false discovery rate.
6.3. Computation of False Omission Rate (FOR)
The false omission rate for newborns in congenital heart disease is approximately 0.2%, which is slightly higher than the general population. This means that there is a 0.2% chance of a newborn with congenital heart disease not being correctly identified and treated. The false omission rate (FOR) of newborns with congenital heart disease is estimated to be around 25-30%. This means that 25-30% of cases of congenital heart disease in newborns could be missed or misdiagnosed.
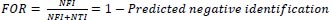 |
(3) |
| No. of Inputs | MLMPR (TR) | MLMPR (TS) | DLIRP (TR) | DLIRP (TS) | AIDLM (TR) | AIDLM (TS) |
|---|---|---|---|---|---|---|
| 100 | 42.43 | 33.54 | 28.10 | 41.13 | 75.09 | 82.79 |
| 200 | 30.22 | 22.19 | 22.68 | 33.84 | 84.01 | 86.08 |
| 300 | 52.02 | 42.44 | 47.65 | 67.36 | 73.77 | 76.95 |
| 400 | 62.68 | 52.34 | 54.31 | 76.31 | 83.44 | 85.18 |
| 500 | 35.87 | 27.45 | 51.83 | 72.98 | 79.14 | 82.12 |
| 600 | 61.74 | 51.47 | 49.31 | 69.61 | 82.59 | 89.01 |
| 700 | 72.63 | 61.59 | 47.79 | 67.56 | 82.46 | 87.13 |
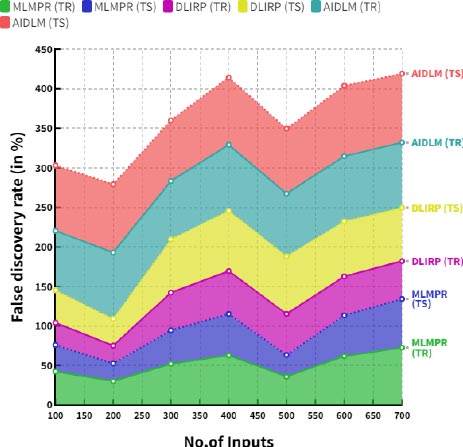
Table 5 provides the comparison of false omission rate (training and testing) between the existing and proposed models.
Fig. (8) demonstrates the comparison of false omission rate between the existing and proposed models. In a comparison tip, proposed AI induced deep learning model (AIDLM) reached 78.48% of training false omission rate and 84.72% of testing false omission rate. In the same range the existing Machine Learning Model for Predicting Risk (MLMPR) has reached 71.85% of training false omission rate and 42.63% of testing false omission rate and Deep learning for improved risk prediction (DLIRP) has obtained 58.86% of training false omission rate and 75.60% of testing false omission rate.
6.4. Computation of Positive Likelihood Ratio (PLR)
The positive likelihood ratio (LR+) for newborns in congenital heart disease is approximately 5.0. This means that a positive test result is five times more likely to be associated with the presence of congenital heart disease in newborns than in those without the condition.
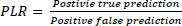 |
(4) |
Table 6 provides the comparison of Positive likelihood ratio (training and testing) between the existing and proposed models.
Fig. (9) demonstrates the comparison of Positive likelihood ratio between the existing and proposed models. In a comparison tip, proposed AI induced deep learning model (AIDLM) reached 70.26% of training Positive likelihood ratio and 82.40% of testing Positive likelihood ratio. In the same range the existing Machine Learning Model for Predicting Risk (MLMPR) has reached 80.18% of training Positive likelihood ratio and 45.02% of testing Positive likelihood ratio and Deep learning for improved risk prediction (DLIRP) has obtained 76.65% of training Positive likelihood ratio and 63.77% of testing Positive likelihood ratio.
| No. of Inputs | MLMPR (TR) | MLMPR (TS) | DLIRP (TR) | DLIRP (TS) | AIDLM (TR) | AIDLM (TS) |
|---|---|---|---|---|---|---|
| 100 | 75.00 | 58.87 | 51.66 | 65.94 | 81.71 | 95.82 |
| 200 | 73.47 | 58.10 | 50.59 | 64.51 | 80.61 | 84.50 |
| 300 | 73.02 | 43.85 | 49.15 | 62.57 | 77.21 | 86.72 |
| 400 | 71.85 | 42.63 | 58.86 | 75.60 | 78.48 | 84.72 |
| 500 | 78.71 | 38.12 | 58.15 | 74.65 | 72.02 | 93.84 |
| 600 | 77.07 | 36.81 | 57.44 | 73.70 | 80.15 | 92.97 |
| 700 | 74.88 | 41.16 | 56.74 | 72.75 | 86.37 | 92.09 |
6.5. Computation of Negative Likelihood Ratio (NLR)
The negative likelihood ratio for newborns with congenital heart disease is 0.18. This means that the likelihood of the diagnosis being false is 18 times higher than the likelihood of it being true.
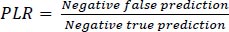 |
(5) |
Table 7 provides the comparison of the negative likelihood ratio (training and testing) between the existing and proposed models.
| No. of Inputs | MLMPR (TR) | MLMPR (TS) | DLIRP (TR) | DLIRP (TS) | AIDLM (TR) | AIDLM (TS) |
|---|---|---|---|---|---|---|
| 100 | 83.70 | 62.15 | 67.27 | 83.76 | 89.10 | 96.03 |
| 200 | 81.99 | 61.35 | 65.89 | 81.93 | 87.91 | 94.81 |
| 300 | 81.49 | 46.29 | 64.01 | 79.48 | 75.66 | 83.61 |
| 400 | 80.18 | 45.02 | 76.65 | 63.77 | 70.26 | 82.40 |
| 500 | 87.83 | 40.25 | 75.73 | 56.72 | 81.65 | 86.03 |
| 600 | 86.01 | 38.87 | 74.80 | 54.68 | 84.68 | 94.81 |
| 700 | 83.56 | 43.46 | 73.88 | 61.47 | 79.85 | 93.61 |
| No. of Inputs | MLMPR (TR) | MLMPR (TS) | DLIRP (TR) | DLIRP (TS) | AIDLM (TR) | AIDLM (TS) |
|---|---|---|---|---|---|---|
| 100 | 81.53 | 42.62 | 62.03 | 76.88 | 80.23 | 82.09 |
| 200 | 59.05 | 47.68 | 61.45 | 76.13 | 77.71 | 91.43 |
| 300 | 57.06 | 46.79 | 60.42 | 74.77 | 76.39 | 90.22 |
| 400 | 77.07 | 36.81 | 57.44 | 73.70 | 80.15 | 82.97 |
| 500 | 74.88 | 41.16 | 56.74 | 72.75 | 76.37 | 82.09 |
| 600 | 73.06 | 40.37 | 47.63 | 60.53 | 75.23 | 90.84 |
| 700 | 52.91 | 45.16 | 47.19 | 59.94 | 72.09 | 90.29 |
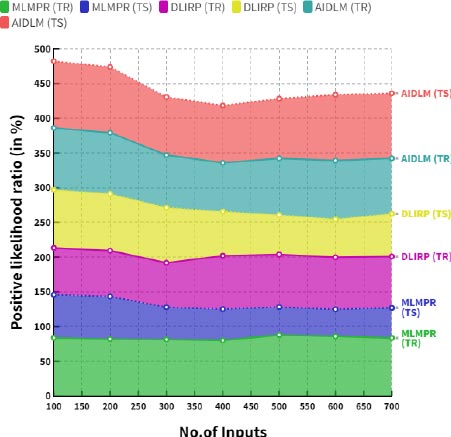
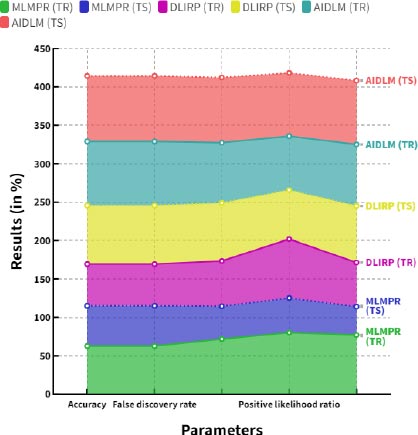
Fig. (10) demonstrates the comparison of negative likelihood ratio between the existing and proposed models. In a comparison tip, the proposed AI induced deep learning model (AIDLM) reached 80.15% of the training negative likelihood ratio and 82.97% of testing negative likelihood ratio. In a same range the existing Machine Learning Model for Predicting Risk (MLMPR) has reached 77.07% of training negative likelihood ratio and 36.81% of testing negative likelihood ratio and Deep learning for improved risk prediction (DLIRP) has obtained 57.44% of training negative likelihood ratio and 73.70% of testing negative likelihood ratio. Table 8 provides the overall comparison between the existing and proposed models.
Fig. (11) shows the Overall comparison between the proposed and existing models. The proposed AI induced deep learning model (AIDLM) reached 68.41% of training accuracy and 84.83% of testing accuracy, 83.44% of training false discovery rate and 85.18% of testing false discovery rate, 78.48% of training false omission rate and 84.72% of testing false omission rate, 70.26% of training Positive likelihood ratio and 82.40% of testing Positive likelihood ratio and 80.15% of training negative likelihood ratio and 82.97% of testing negative likelihood ratio.
In a comparison tip, the proposed In addition to its potential in screening, deep learning algorithms can also be used to accurately differentiate between true CHD cases and false positives. This can help healthcare providers focus their resources on the cases that require the most attention. Deep learning algorithms can also be used to detect the specific gene mutations that are associated with CHD, allowing healthcare professionals to tailor treatments to the specific needs of the patient. Deep learning algorithms can be used in combination with other diagnostic tests, such as echocardiograms, to improve the accuracy of CHD diagnosis and treatment. Deep learning algorithms can also be used in conjunction with other medical data, such as family and medical history, to detect signs of CHD before it is too late. Overall, deep learning algorithms offer a powerful tool for the early detection and treatment of CHD. By leveraging the latest technology, healthcare providers can diagnose and treat this condition more effectively, improving the prognosis for their patients.
| Parameters | MLMPR (TR) | MLMPR (TS) | DLIRP (TR) | DLIRP (TS) | AIDLM (TR) | AIDLM (TS) |
|---|---|---|---|---|---|---|
| Accuracy | 62.68 | 52.34 | 54.31 | 76.31 | 83.44 | 85.18 |
| False discovery rate | 62.68 | 52.34 | 54.31 | 76.31 | 83.44 | 85.18 |
| False omission rate | 71.85 | 42.63 | 58.86 | 75.60 | 78.48 | 84.72 |
| Positive likelihood ratio | 80.18 | 45.02 | 76.65 | 63.77 | 70.26 | 82.40 |
| Negative likelihood ratio | 77.07 | 36.81 | 57.44 | 73.70 | 80.15 | 82.97 |
CONCLUSION
The use of deep learning for newborn screening of congenital heart disease (CHD) has the potential to revolutionize the way healthcare providers diagnose and treat this condition. Deep learning algorithms can be used to analyze a baby’s heart rate, rhythm, and other vital signs in order to identify possible signs of CHD. These algorithms can be trained to recognize signs that are often missed by traditional tests, such as subtle changes in the heart rate or abnormal rhythms. Deep learning algorithms can also be used to detect abnormalities in the fetal heart rate that are difficult to detect with manual techniques. Using deep learning for CHD screening can reduce the time and resources needed to diagnose and treat this condition. By using machine learning algorithms, healthcare providers can quickly identify possible CHD cases and begin treatment earlier. This not only improves the prognosis of the patient but also reduces the cost of care. Furthermore, deep learning algorithms can be used to identify high-risk pregnancies and intervene early to reduce the complications of CHD. The proposed model reached 68.41% of training accuracy and 84.83% of testing accuracy, 83.44% of training false discovery rate and 85.18% of testing false discovery rate, 78.48% of training false omission rate and 84.72% of testing false omission rate, 70.26% of training Positive likelihood ratio and 82.40% of testing Positive likelihood ratio and 80.15% of training negative likelihood ratio and 82.97% of testing negative likelihood ratio. The proposed deep learning approach can be used to help diagnose newborns with congenital heart disease. Deep learning models can be trained to identify patterns in medical images, such as echocardiograms and ultrasounds, that are characteristic of certain types of heart conditions. This can help to quickly and accurately diagnose complex heart conditions in newborns, as well as provide additional information, such as the severity of a condition and the best treatment options. Additionally, deep learning can be used to analyze the genetic material of newborns to look for mutations associated with certain types of congenital heart disease. This could help to identify conditions earlier, allowing for more timely and effective interventions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No humans and animals were used in the study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Vinayakumar Ravi is the Associate Editorial Board Member of the journal The Open Bioinformatics Journal.
ACKNOWLEDGEMENTS
Declared none.


